Which of the Following Was Not Among Democritus's Ideas
We can not describe the internal structure of the atom itself. Except as contradictions to one another.

1 Chapter 4 Atomic Structure 2 Section 4 1 Defining The Atom Objectives Objectives Describe Democritus S Ideas About Atoms Describe Democritus S Ideas Ppt Download
Which of the following was originally a tenet of Daltons atomic theory but had to be revised about a century ago.

. That there is an infinite number of atoms and of kinds of atoms which differ in shape and size. Which of the following was originally a tenet of daltons atomic theory but had to be revised about a century ago. Aristotle believed that objects were a continuum and he strongly objected to the.
The theory of Democritus held that everything is composed of atoms which are physically but not geometrically indivisible. Which of the following was NOT among Democrituss ideas. And was among the first to suggest the idea of atoms.
View ch4docx from MATH 54 at Yale University. The first proponents of an atomic theory were the Greek philosophers Leucippus and Democritus who proposed the following model in the fifth century BC. Matter consists of tiny particles called atoms.
Democritus was not able to describe atomic model in detail. See answer 1 Best Answer. Matter is composed of atoms separated by empty space through which the atoms move.
Which of the following was NOT among Democrituss ideas. Matter consists of tiny particles called atoms b. Atoms retain their identity in a chemical reaction.
A Matter consists of tiny particles called atoms. Atoms retain their identity in a chemical reaction. D Atoms are indestructible.
Which of the following was NOT among Democrituss ideas. Atomic theory is the scientific theory that describes the nature of. All matter is composed of tiny particles that can vary in shape size and weight.
That atoms are indestructible and have always been and always will be in motion. Which of the following was not among democrituss ideas. Atoms retain their identity in a chemical reaction d.
B Atoms are indivisible. Atoms retain their identity in a chemical reaction. C Atoms retain their identity in a chemical reaction.
Which of the following is NOT a part of Daltons atomic theory. Atoms are tiny indivisible particles. Which of the following was NOT among Democrituss ideas.
Democritus known in antiquity as the laughing philosopher because of his emphasis on the value of cheerfulness was one of the two founders of ancient atomist theory. Atoms are indivisible c. Atoms retain their identity in a chemical reaction.
Matter consists of tiny particles called atoms. Group of answer choices atoms are indestructible. 160Who was the man who lived from 460BC370BC.
On his theory Democritus only stated that atoms are in the solid form in the void sphare. A few decades after Empedocles Democritus 460 BCE - 370 BCE who was also Greek developed a new theory of matter that attempted to overcome the problems of his predecessor. He elaborated a system originated by his teacher Leucippus into a materialist account of the natural world.
Atoms retain their identity in a chemical reaction. Which of the following was NOT among Democrituss ideas. Which of the following was NOT among Democrituss ideas.
Matter consists of tiny particles called atoms. We now know that Atoms consist of 3 parts which are proton neutron and electron. 1 point Matter consists of tiny particles called atoms.
Democrituss ideas were based on reasoning rather than science and drew on the teachings of two Greek philosophers who came before him. C Atoms retain their identity in a chemical reaction. B Atoms are indivisible.
Which of the following was NOT among Democrituss ideas. Unfortunately this monograph has been lost. Well they dont relate.
That between atoms there lies empty space. The key difference between Democritus and Dalton atomic theory is that the Democritus atomic theory is an ancient theory that scientists later refined and elaborated whereas Dalton atomic theory is a comparatively modern scientific theory that we cannot discard due its important statements. D Atoms are indestructible.
The atom is proposed. Atoms are solid homogeneous indivisible and unchangeable. Aristotle did take Democritus seriously and wrote a monograph criticizing his theory.
Answer 1 of 3. A Matter consists of tiny particles called atoms.

Atomic Thoeries Science Quizizz

Chapter 4 Atomic Structure Define Democritus S Ideas About Atoms Explain Dalton S Atomic Theory Identify What Instrument Is Used To Observe Individual Ppt Download

Solved 1 Which Of The Following Was Not Among Democritus S Chegg Com

The Evolution Of The Model Of The Atom Sutori

Chapter 4 Atomic Structure Define Democritus S Ideas About Atoms Explain Dalton S Atomic Theory Identify What Instrument Is Used To Observe Individual Ppt Download
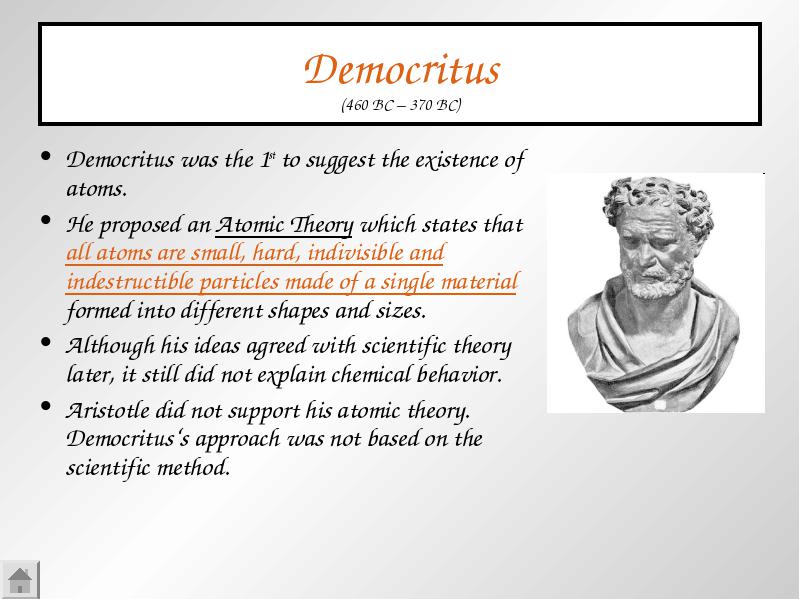
Democritus Was The 1st To Suggest The Existence Of Atoms Democritus Was The 1st To Suggest The Existence Of Atoms

Which Of The Following Was Not Among Democritus S Ideas Brainly Com

P 122 Q 35 What Were The Limitations Of Democritus S Ideas Ppt Download

Unit 1 The Atom Atomic Theory The Nucleus

Main Ideas 1 Atom 2 Dalton S Atomic Theory Key Concepts 1 How Did Democritus Describe Atoms 2 How Did John Dalton Further Democritus S Ideas On Atoms Ppt Download

The Structure Of The Atom Ppt Download

Atomic Thoeries Science Quizizz

Unit 2 Atomic Structure Nuclear Chemistry New Book

1 Chapter 4 Atomic Structure 2 Section 4 1 Defining The Atom Objectives Objectives Describe Democritus S Ideas About Atoms Describe Democritus S Ideas Ppt Download





Comments
Post a Comment